Ten-year Retrospective Study on the Efficacy of a Manual Physical Therapy to Treat Female Infertility
Amanda D. Rice, PhD; Kimberley Patterson, PTA; Leslie B. Wakefield, DPT; Evette D. Reed, PT; Kelseanne P. Breder, BA; Belinda F. Wurn, PT; C. Richard King III, MD; Lawrence J. Wurn, LMT
Amanda D. Rice, PhD, is the director of clinical studies; Kelseanne P. Breder, BA, is an intern. Both are in the research department at Clear Passage Physical Therapy in Gainesville, Florida. Kimberley Patterson, PTA, is a therapist; Leslie B. Wakefield, DPT, is a physical therapist; Evette D. Reed, PT, is a physical therapist; and Belinda F. Wurn, PT, is a clinical director. All are located in the clinical department at Clear Passage Physical Therapy. C. Richard King, III, MD, is the medical director of Clear Passage Physical Therapy. Lawrence J. Wurn, LMT, is a therapist in the clinical department and the research director in the research department at Clear Passage Physical Therapy.
ABSTRACT
Background • Female infertility is a complex issue encompassing a wide variety of diagnoses, many of which are caused or affected by adhesions.
Objectives • The study intended to examine the rates of successful treatment of infertile women using a protocol of manual physical therapy to address underlying adhesive disease leading to infertility.
Methods • The research team designed a retrospective chart review.
Setting • The study took place in a private physical therapy clinic.
Participants • Participants were 1392 female patients who were treated at the clinic between the years of 2002 and 2011. They had varying diagnoses of infertility, including occluded fallopian tubes, hormonal dysfunction, and endometriosis, and some women were undergoing in vitro fertilization (IVF).
Intervention • All patients underwent whole-body, patient-centered treatments that used a protocol of manual physical therapy, which focused on restoring mobility and motility to structures affecting reproductive function.
Outcome Measures • Improvements demonstrated in the condition(s) causing infertility were measured by improvements in tubal patency and/or improved hormone levels or by pregnancy.
Results • The results included a 60.85% rate of clearing occluded fallopian tubes, with a 56.64% rate of pregnancy in those patients. Patients with endometriosis experienced a 42.81% pregnancy rate. The success rate was 49.18% for lowering elevated levels of follicle stimulating hormone (FSH), with a 39.34% pregnancy rate in that group, and 53.57% of the women with polycystic ovarian syndrome (PCOS) achieved pregnancy. The reported pregnancy rate for patients who underwent IVF after the therapy was 56.16%. The results also suggested that the treatment was effective for patients with premature ovarian failure (POF). Conclusion • The manual physical therapy represented an effective, conservative treatment for women diagnosed as infertile due to mechanical causes, independent of the specific etiology. (Altern Ther Health Med. 2015;21(3):32-40.)
Infertility affects 6% to 11% of women in the United States,1–3 stemming from a wide variety of diagnosed medical conditions. These rates are similar to those of other developed countries.4 The most common etiologies for female infertility include occluded fallopian tubes, endometriosis, elevated levels of follicle stimulating hormone (FSH) that signal a decrease in ovarian function, and polycystic ovarian syndrome (PCOS). Among the less common causes of infertility in women are premature ovarian failure (POF) and unexplained infertility, a classification in which no cause for infertility can be identified. To date, no single treatment has proven effective in producing natural conception in women with this broad spectrum of infertility etiologies. However, a variety of treatment options with varying degrees of invasiveness exist for each condition, from a wait-and-see approach to surgery or in vitro fertilization (IVF).
Manual manipulation therapy is used by physical therapists, osteopathic physicians, and chiropractors as a standard treatment in a wide variety of medical diagnoses, such as adhesive capsulitis, pain from post mastectomy scars, back pain, and dysfunction related to the pelvic floor.5–13 The intent of manual physical therapy is to decrease pain and increase mobility; its use is applicable to all areas of the body, from osseous structures to soft tissue, including skin and muscles.14,15
In the current 10-year retrospective analysis, a single treatment protocol, the Clear Passage Approach (CPA), demonstrated efficacy in treating female infertility that was due to a variety of etiologies. The study examined the success rates for various conditions when using the protocol, which combines several whole-body and site-specific techniques of manual physical therapy. That protocol focuses on deforming the adhesive collagen cross-links that comprise adhesions and appear to contribute to the underlying causes of infertility, including mechanical blockages and some hormonal imbalances. Decreasing adhesions that bind the organs appears to help the body to function and to respond in a more appropriate physiological manner.
METHODS
Participants
Patients treated for infertility between 2002 and 2011 at Clear Passage Physical Therapy’s (CPPT’s) sites were included in the current retrospective study. All sites included are private physical therapy practices. Infertility was defined based on criteria from the Centers for Disease Control and Prevention (CDC) as the inability to conceive for 12 months of unprotected intercourse in women younger than age 35 years or for 6 months in women older than age 35 years.2 The physicians’ diagnoses and the patients’ self-reported medical histories were examined for causes of infertility, including elevated FSH levels, blocked fallopian tubes, endometriosis, PCOS, POF, and unexplained infertility. Although most successes noted in this study were via natural conception, the study also included women who had therapy prior to IVF, by separate reference.
To be included in the study, the patients must have had a previous diagnosis of infertility prior to treatment, with a corresponding medical cause(s). Classifications of patients and the measures of success were defined for each of the 6 reported etiologies of infertility: (1) elevated FSH, (2) tubal occlusion, (3) endometriosis, (4) PCOS, (5) POF, and (6) unexplained infertility.
Elevated FSH. For the purpose of the current study, elevated FSH was defined as a level of 10 mIU/mL or higher on days 2 through 5 of the menstrual cycle. All testing for FSH levels was performed in independent, nationally regulated clinical laboratories using standardized references and protocols.16 Success was defined as a decrease in FSH levels post treatment, as assessed at days 2 through 5 of the menstrual cycle and/or pregnancy.
Tubal Occlusion. The patients were those women with total tubal occlusion prior to treatment, including those with only 1 fallopian tube, as diagnosed by either surgery or hysterosalpingogram (HSG). In patients with bilateral tubal occlusion, treatment was considered to be successful if a post treatment HSG demonstrated open fallopian tube(s) or if intrauterine pregnancy was confirmed, with the assumption that at least 1 tube was opened during treatment.
Endometriosis, PCOS, POF, or Unexplained Infertility. Treatment of patients with a medical history of endometriosis, PCOS, POF, or unexplained infertility was considered a success if they experienced a confirmed pregnancy.
In Vitro Fertilization. Patients who underwent the CPA treatment prior to IVF were considered a success if they had a positive clinical pregnancy test post-IVF, regardless of the source of implanted egg/embryos—fresh or frozen, donor or nondonor eggs.
The rates of success for patients that underwent the CPA treatment were compared with those in published studies in which the rates of success from standard medical treatments were reported for each diagnosis. All patients consented to the use of their data in a standard form for informed consent, and the study was approved by MaGil IRB (Rockville, MD, USA).
A subset of the patients included in the 10-year analysis had also been included in small, multiple case reports or reports of phase I studies; a total of 76 of the 1392 patients had been included in those previous reports.17–21
Follow-up
Previous patients were contacted for follow-up after their treatment at CPPT using questionnaires that were sent annually by postal mail or e-mail, and they were questioned regarding pregnancies, pregnancy outcomes, and all efforts to achieve pregnancy. Patients treated for elevated FSH were asked to provide their post treatment FSH levels. Patients lost to follow-up were not included in the analysis.
Treatment
Patients were treated using a patient-centered, individualized treatment plan of physical therapy that is standard practice at the clinic and that included a comprehensive review of the patient’s medical history and an initial evaluation. The regimen and modalities included in the treatment plan are termed the CPA, which uses a variety of recognized techniques of manual physical therapy, together with refinements and modifications of those techniques. All physical therapists had been trained and certified in the CPA; therefore, the variation in treatment was negligible between therapists. The techniques that the therapy used had been examined previously, with results published by the research group and others.15,17–20,22–31
Based on each patient’s goals and subjective complaints and on findings from the initial evaluation, the CPA protocol was individualized to treat sites that presented with restricted mobility within each patient’s body.32 The therapy used various site-specific pressures across the restrictive bands of adhered tissues and structures, working progressively deeper from the most superficial tissues, to restore mobility via myofascial release.33 Adhesions within and between organs and interstitial spaces within the viscera were addressed using the Wurn technique29; decreased organ motility was then addressed using visceral manipulation.33 The amount of force and the length of time that the force was applied to each area had the potential to be significant, but the treatment was maintained within the tolerance of the patient. The standard treatment regimen was 20 hours, administered 4 hours per day for 5 days.
Statistical Analysis
Basic descriptive statistics were generated based on data from the clinic’s database. Correlations were identified using DataDesk version 6.2.1 (Data Descriptions, Ithaca, NY, USA), via a linear regression model in which items from past medical history were used as the variables against a determination of a positive or negative outcome for each measured condition. A P ≤ .05 was considered suggestive, and P < .005 was considered significant. All statistical analyses were completed on the data as a whole as well as separately for variations by site or therapist; no variation was identified (data not shown).
Standard methods for odds ratio calculations were used for the CPA treatment’s success rates compared with those of published studies.34 Significance was determined to be P ≤ .05 where the odds ratio confidence interval did not include 1.0, the point of equivalence.
RESULTS
For the study, 1392 patients were identified for individual infertility conditions based on previous medical history, including physicians’ diagnoses. Each condition was analyzed separately, with patients experiencing multiple conditions being included in all analyses for which they were identified. In addition to each condition being assessed separately, the influence of age, year of treatment, treating therapist, and past medical history were analyzed for predictors of outcomes, both negative and positive. The year and treating therapist did not influence outcomes in any analysis (data not shown).
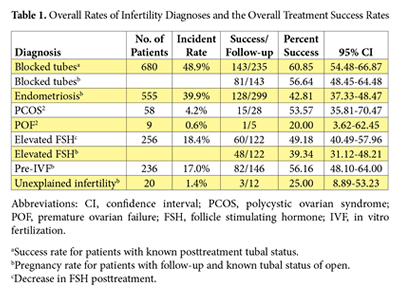 Of the patients included in the study, 428 (30.7%) exhibited multiple conditions and were represented in more than 1 categorical analysis. Although some patients were included as a success in more than 1 analysis, this finding was not viewed as a negative given the overall percentage of women included in multiple analyses, assuming that multiple diagnoses of infertility for any patient would not improve her chances of success and that no additive effect occurred for the numbers or types of infertility diagnoses. The number of patients and relative incidence rates for each category are shown in Table 1.
Of the patients included in the study, 428 (30.7%) exhibited multiple conditions and were represented in more than 1 categorical analysis. Although some patients were included as a success in more than 1 analysis, this finding was not viewed as a negative given the overall percentage of women included in multiple analyses, assuming that multiple diagnoses of infertility for any patient would not improve her chances of success and that no additive effect occurred for the numbers or types of infertility diagnoses. The number of patients and relative incidence rates for each category are shown in Table 1.
Blocked Fallopian Tubes
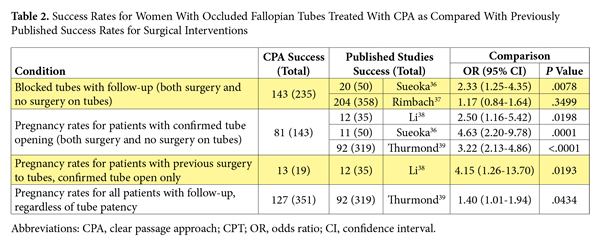
Blocked fallopian tubes, estimated as the primary cause of 25% to 35% of female infertility,35 were observed in 680 of the treated patients, with follow-up in 235 patients. Being the largest group treated at the clinics for infertility, several outcomes were measured. The first outcome was the success rate for the return to patency of at least 1 fallopian tube post- CPA treatment, which was a 60.85% (95% CI, 54.48-66.87). The standard-of-care option for women with blocked fallopian tubes to open their tubes is surgery, with several surgical options available depending on the location of the blockage. When the rates of patients with successfully opened fallopian tubes post-CPA treatment were compared with reported rates of success from the literature, it was observed that the CPA performed as well as or at higher rates of success than surgery did (Table 2).
Upon examining predictors for a positive outcome, previous surgery to open the fallopian tubes was a statistically significant predictor of a negative outcome with P ≤ .0001. When the patients were analyzed separately based on a history of previous surgery to open their tubes, the success rate was 68.9% (124/180) for patients with no history of surgery and 34.5% (19/55) for patients whose surgical history involved attempts to open their tubes. The type of surgery performed on the fallopian tubes could not analyzed as a predictor of outcomes due to the lack of information regarding the actual procedures performed for each patient, related either to incomplete data on or unavailability of some surgical reports.
Suggestive predictors for positive outcomes (ie, open fallopian tubes post-CPA) included (1) reporting of prior physical or sexual abuse, P = .0085; (2) current neck pain, P = .0124; (3) neurological conditions, P = .0312; (4) prior sexually transmitted disease (STD), P = .0345; and (5) prior pelvic inflammatory disease, P = .0501. Suggestive predictors for negative outcomes were (1) Crohn’s disease, P = .0380; (2) diagnosis of pelvic adhesions, P = .0139; and (3) previous laparotomy, P = .0101.
The location and characteristics of each type of fallopian tube occlusion—proximal versus distal or midtubal occlusion and the presence of a hydrosalpinx—were analyzed to determine outcome predictors for successful treatment. Neither location of tubal occlusion nor presence of a hydrosalpinx was a significant predictor for outcomes (P = .0429). The rate of resolution of hydrosalpinx was 44.8%; 47/105 total tubes with hydrosalpinx before the CPA treatments were patent after therapy, with no evidence of hydrosalpinx.
The rate of pregnancy for patients with successful treatment resulting in at least 1 open fallopian tube was also determined, independent of prior history of fallopian tube surgery. The overall pregnancy rate was 56.64% (81/143) for patients who had at least 1 open fallopian tube after the CPA treatment. One pregnancy was via IVF, with all other pregnancies via natural conception. The single patient who underwent IVF after the CPA treatment had opened her fallopian tubes experienced a subsequent natural pregnancy following the successful IVF, suggesting that the results of the CPA treatment for opening blocked fallopian tubes are long-lasting. In fact, several women who presented bilateral tubal occlusion before treatment reported multiple natural pregnancies after treatment.
Prior tubal surgery did not influence pregnancy rates in women for whom the CPA treatment was successful in opening their fallopian tubes. Pregnancy rates from patients with a known tubal status after the CPA treatment were compared with published rates of pregnancy in women who had surgical interventions for blocked tubes.
Again, the CPA treatment was demonstrated to have rates that were comparable to or higher than surgical interventions (Table 2). Patients for whom treatment did not open at least 1 fallopian tube and who did become pregnant via IVF post treatment were also included in the pre-IVF statistics.
Endometriosis
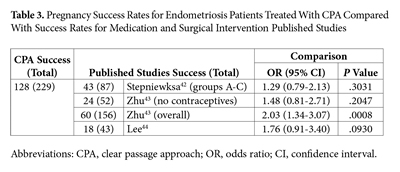 Endometriosis is present in approximately 11% of the female population40 but is the cause of infertility in 20% to 68% of infertile women.41 Of the patients treated with the CPA, 558 had a previous diagnosis of endometriosis from a physician. More than one-half of those patients also presented with additional conditions, such as blocked fallopian tubes and elevated FSH. In the endometriosis analysis, success was defined as pregnancy; the overall success rate was 42.8%—128 of 299 patients with follow-up. The study included 214 patients with endometriosis as the only diagnosed condition leading to infertility, with follow-up on 118 patients and a pregnancy rate of 37.3% (44/118). The standard medical treatments for endometriosis are varied, depending on the goals and the needs of the individual patient. Treatments may include surgical intervention to remove the endometriosis from the pelvic cavity, contraceptives, or combinations of these therapies. When compared with published success rates for pregnancy in women with endometriosis who were treated with standard medical interventions, the CPA demonstrated rates similar to or better than standard of care (Table 3).
Endometriosis is present in approximately 11% of the female population40 but is the cause of infertility in 20% to 68% of infertile women.41 Of the patients treated with the CPA, 558 had a previous diagnosis of endometriosis from a physician. More than one-half of those patients also presented with additional conditions, such as blocked fallopian tubes and elevated FSH. In the endometriosis analysis, success was defined as pregnancy; the overall success rate was 42.8%—128 of 299 patients with follow-up. The study included 214 patients with endometriosis as the only diagnosed condition leading to infertility, with follow-up on 118 patients and a pregnancy rate of 37.3% (44/118). The standard medical treatments for endometriosis are varied, depending on the goals and the needs of the individual patient. Treatments may include surgical intervention to remove the endometriosis from the pelvic cavity, contraceptives, or combinations of these therapies. When compared with published success rates for pregnancy in women with endometriosis who were treated with standard medical interventions, the CPA demonstrated rates similar to or better than standard of care (Table 3).
Endometriosis is recognized as a cause of fallopian tube occlusion, often associated with the formation of pelvic adhesions. For the 228 patients treated for both endometriosis and occluded fallopian tube(s), the current analysis found a pregnancy rate of 38.1% (45/118). For women with endometriosis and fallopian tube occlusion who had post treatment documentation of tubal patency status, the success rate for opening blocked fallopian tubes was 59.7% (40/67). The rates of success in patients with prior surgery to open their fallopian tubes were similar to those reported in the section providing statistics on blocked fallopian tubes.
A subset of patients with endometriosis underwent the CPA treatment prior to the initiation of IVF, independent of tubal patency or other outcomes. Of the 56 patients with follow-up who underwent IVF post-CPA treatment, the clinical pregnancy rate after transfer was 55.4% (31/56), which is 1.3 times that of the national average of 40.3% for IVF transfer alone in women with endometriosis.45 This subset of patients was included in the overall statistics for IVF success.
No pregnancy was reported for either of the 2 treated patients who had both POF and endometriosis. Women with both PCOS and endometriosis had a pregnancy rate of 75% (6/8), with all pregnancies reported via natural conception. Women with endometriosis and elevated FSH had a pregnancy rate of 40% (10/25), with 2 pregnancies via IVF and 8 by natural conception. The numbers of women in these subcategories were not sufficient to have adequate statistical comparisons with other treatments but do support the initiation of future studies involving these comorbid conditions.
Predictors for outcomes of treatment for women with endometriosis were also performed using linear regression. Age was identified as a significant predictor (P = .001), where women younger than age 35 years were more likely to achieve pregnancy. A diagnosis of fibromyalgia was identified as a suggestive negative predictor (P = .0180).
Elevated Follicle Stimulating
Hormone
Between 2002 and 2011, 250 women were treated at the research team’s clinics for elevated FSH, as defined at a level of 10 mIU/mL or higher on days 2 through 5 of the menstrual cycle. The overall success rate for women with elevated FSH, as defined by either a decrease in FSH levels or a pregnancy was 49.18% (60/122). The pregnancy rate was 39.34% (48/122), with 43 natural and 5 IVF pregnancies. No medical treatment that represents standard of care currently exists for women with elevated FSH levels; therefore, no comparisons with standard of care could be performed.
Although no statistically significant predictors for outcomes were identified in the analysis, a higher pregnancy rate was observed in women younger than age 35 years than in women older than age 35 years.
Premature Ovarian Failure
POF has a 1% to 2% prevalence rate in the general population46 and is characterized by an increase in FSH and a decrease in ovarian function, with or without amenorrhea. Several studies have reported a spontaneous pregnancy rate of 4% to 10% in patients with POF, in which ovulation and normal menses occur unpredictably.47,48 Between 2002 and 2011, 9 patients with POF were treated in the research team’s clinics using the CPA, and follow-up occurred with 5 patients. One patient became pregnant, giving an overall success rate of 20%. That patient conceived naturally, with no assistive technology or ovulation-inducing medications. Because she was 30 years old, the success of the patient may have been due in part to her age, whereas the other patients were aged 38 years or older. Therefore, the length of time with elevated FSH and ovarian failure might have been less likely to affect successful treatment than for the other patients treated with POF. As so few patients were in this group, analysis for identification of predictors for outcomes could not be performed, and as no medical treatment for infertility in these patients exists, no comparisons could be performed.
Polycystic Ovarian Syndrome
PCOS affects an estimated 8% to 20% of women worldwide.49,50 Anovulation is commonly associated with PCOS patients, directly affecting fertility.49,51,52 A total of 59 patients with a history of PCOS were treated with the CPA, with follow-up available for 28 patients. The overall pregnancy success rate was 53.57% (15/28); one of the 15 pregnancies in this data subset was achieved via IVF.
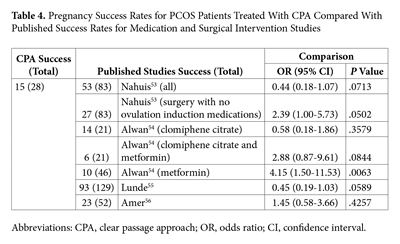 Options for standard medical treatment of PCOS include medications—clomiphene citrate, metformin, or combinations thereof—and surgical interventions to induce ovulation. The rates of pregnancy in patients treated using the CPA were directly compared with success rates for various interventions reported in the literature. The CPA demonstrated significantly higher rates of pregnancy when compared with metformin alone; the CPA treatment presented no significant differences in rates of pregnancy when compared with clomiphene citrate or the surgical interventions of ovarian wedging or drilling (Table 4).
Options for standard medical treatment of PCOS include medications—clomiphene citrate, metformin, or combinations thereof—and surgical interventions to induce ovulation. The rates of pregnancy in patients treated using the CPA were directly compared with success rates for various interventions reported in the literature. The CPA demonstrated significantly higher rates of pregnancy when compared with metformin alone; the CPA treatment presented no significant differences in rates of pregnancy when compared with clomiphene citrate or the surgical interventions of ovarian wedging or drilling (Table 4).
Predictors for outcomes for PCOS patients were again analyzed by linear regression. No significant predictors were identified; however, several predictors were identified as suggestive of success, with P values lower than .05. Suggestive negative predictors for pregnancy were (1) previous vaginal infections, P = .0254; (2) a prior STD infection, P = .0238; and (3) reported back pain, P = .0130. Suggestive positive predictors were identified as (1) dyspareunia, P = .0110, and (2) dysmenorrhea, P = .0075. Although not significant in the analysis, patients with prior surgery to correct anovulation— ovarian wedging or drilling—did not report overall improvement levels that were similar to patients who had not undergone prior surgery for PCOS, suggesting further studies involving this patient population should be examined.
Unexplained Infertility
Unexplained infertility affects an estimated 18% to 28% of women, with no medical cause for the inability to conceive.57,58 For the 10 years presented in this report, 20 women were treated at the clinic with unexplained infertility, with follow-up with 12 of these patients. These patients experienced a 25% pregnancy rate (3/12). One of the 3 pregnancies was attributed to IVF, whereas the other 2 were via natural conception. These rates are in line with published observational rates of pregnancy in a population with unexplained infertility59; however, the CPA may decrease the time to first conception in these patients and this finding presents a topic for future investigation.
Treatment Prior to IVF
In 2010, more than 135 800 IVF cycles were completed in the United States. The national average for pregnancy after transfer was 37.3% in 2010, as reported by the Society for Assisted Reproductive Technology (SART),45 with a distinct decrease in pregnancy rates by 5-fold for the older-than-age-42 category versus the younger-than-age-35 category.
A total of 231 women were treated with the CPA prior to undergoing IVF. The overall pregnancy rate for the 146 patients with follow-up was 56.16% (82/146), which is 1.5 times that of the national IVF pregnancy rate without the CPA.45 Those data encompassed all infertility diagnoses, including those noted by the research team’s group, allowing for a direct comparison to SART’s overall data. A clear trend and an associate predictor for outcomes by age existed, in which younger patients had higher rates of pregnancy with subsequent IVF cycles. Table 5 shows the comparisons for success rates for women treated with the CPA prior to IVF transfer compared with the national statistics for all transfers by age between 2003 and 2011. The data demonstrate significantly higher rates of success in patients treated with the CPA prior to IVF transfer for all ages.
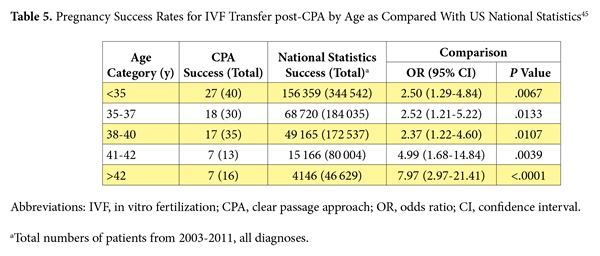
The only suggestive positive predictor for outcomes for the IVF data set as a whole was age (P = .0443). This observation is in line with standard guidance for infertility and current literature in which the younger-than-age-35 category has demonstrated higher pregnancy rates.
DISCUSSION
 Physical therapy is a commonly used medical, adjuvant treatment for a variety of conditions. It is typically focused on one area of the body, with a limited number of modalities. In this article, the research team has demonstrated successful treatment with the CPA of women with various underlying causes for infertility. The CPA uses a combination of manual physical therapy techniques to treat the entire body of each patient, with a focus on decreasing adhesions and cross-links that bound neighboring tissues during a prior healing event. The CPA treatment appeared to improve or restore function for patients with various infertility diagnoses, with a return of fertility and fecundity.
Physical therapy is a commonly used medical, adjuvant treatment for a variety of conditions. It is typically focused on one area of the body, with a limited number of modalities. In this article, the research team has demonstrated successful treatment with the CPA of women with various underlying causes for infertility. The CPA uses a combination of manual physical therapy techniques to treat the entire body of each patient, with a focus on decreasing adhesions and cross-links that bound neighboring tissues during a prior healing event. The CPA treatment appeared to improve or restore function for patients with various infertility diagnoses, with a return of fertility and fecundity.
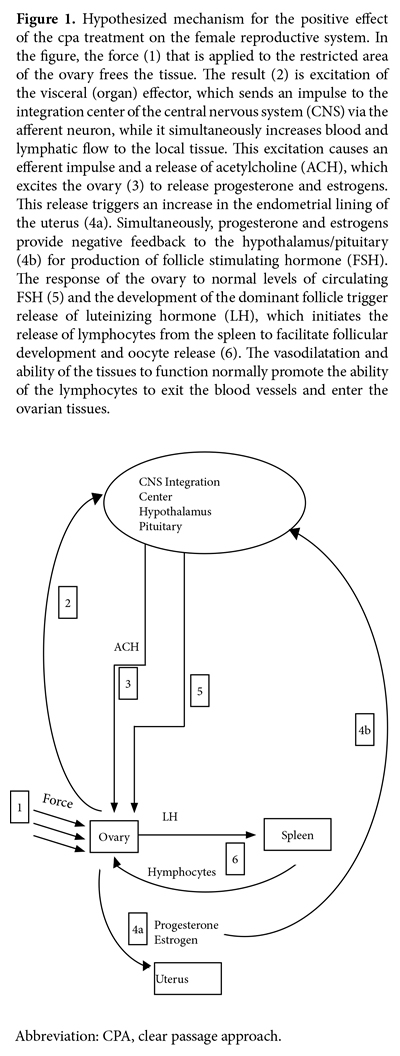
The hypothesized mode of action for the CPA has been developed based on documentation in the literature, clinical observations, and knowledge of mechanisms and modes of action from other manual therapy modalities (Figure 1). The CPA, with an emphasis on the Wurn technique for patients in the current study, appeared to work by deforming adhesions in the body, thereby allowing increased mobility, motility, and function of the structures involved in fertility.
Some causes of infertility are straightforward, such as occluded fallopian tubes, whereas others are quite complex, such as POF and PCOS. In cases of straightforward adhesive disease, dissociation and deformation of the adhesions is sufficient in many cases to restore fertility. However, in more complex etiologies of infertility, including hormonal regulation, the research team’s hypothesis is that the therapy affects 1 or more mechanical blocks in communication between the brain and the reproductive system—the hypothalamic-pituitary-gonadal axis or other mechanical paths. The research team theorizes that these blocks may be caused either by (1) misalignment of the cranium or spine, affecting nervous system communication; (2) dysfunctional tissues surrounding blood vessels; (3) nerves in the pelvic region decreasing efficient stimulation, or (4) a combination of any of these causes. Aberrant ovulation and hormonal function have been observed in women with spinal injuries, supporting the observation that mild spinal misalignment could be sufficient to influence hormonal function.60
Treatment with the CPA had a 60.85% success rate overall for opening 1 or both tubes in women with total tubal occlusion, and the data suggest that the fallopian tubes remained open, indicating that the adhesions did not return unless subsequent inflammation, infection, trauma, or surgery occurred. Prior surgery to treat occluded fallopian tubes was identified as a negative predictor for success of the CPA treatment. This finding is hypothesized to be due to the damage to the fallopian tubes that occurs during surgical procedures, which has been suggested to be the initiating factor for tubal reocclusion in surgical literature.61,62 The rates of success for the CPA, when directly compared with the rates of success for surgical interventions, demonstrated equivalence or superiority for tubal patency post intervention and for subsequent pregnancy. Further studies to assess the impact on the type of surgical procedure and outcomes prior to the CPA treatment are planned.
The use of the CPA in treating women with endometriosis had previously been shown by the research group to be efficacious in the treatment of sexual dysfunction, dyspareunia, and dysmenorrhea, with long-lasting results.18,19,63 The success in using the CPA as a treatment for women experiencing infertility associated with endometriosis has now been demonstrated, with a 42.8% pregnancy rate and equivalence to pregnancy rates after surgical interventions. Given the high rate of success, further studies are warranted to assess the effect of the stage of endometriosis on treatment outcomes.
Hormonal dysregulation impacts a woman’s fertility and is often challenging to treat. The CPA has been shown in the current study to affect hormonal regulation positively in women with POF, PCOS, and elevated FSH, with success rates of 20%, 53.6%, and 49.2%, respectively. The numbers of patients in the POF studies were not adequate to perform stringent analyses; however, further studies are planned given the positive initial results observed. The rate of pregnancy in PCOS patients treated with the CPA, when directly compared with surgical or medication interventions, was again demonstrated to be equivalent or superior to those standard interventions.
The CPA treatment was also demonstrated to be beneficial to those patients who underwent IVF after treatment, with pregnancy rates 1.5 times higher than that for IVF alone, as reported by SART.45 When compared with national statistics for IVF success rates, the CPA was shown to be superior to IVF alone for all age categories.
Limitations of this study include a high rate of drop-out to follow-up and a dependence for a comprehensive medical history upon the patients’ self-reports of that history and on their provision of the surgical and test results. In addition, incomplete data existed on male factors affecting fertility rates in the treated women. Consequently, the actual rates of success for female infertility may be higher than reported here because it is estimated that up to 40% of infertile couples have male factors that contribute to infertility.64,65 Live birth rates were unable to be reported due to low follow-up rates. Additional prospective, controlled studies are planned to examine further the suggestive predictors and comprehensive data for outcomes post-CPA treatment for all etiologies of infertility.
CONCLUSIONS
Manual physical therapy has been demonstrated to reverse female infertility attributed to occluded fallopian tubes, hormonal dysregulation, endometriosis, and unexplained infertility and to increase successful in vitro cycles.
AUTHOR DISCLOSURE STATEMENT
The research team received no funding for this study and have no conflicts of interest related to it.
REFERENCES
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of US women: data from the 2002 National Survey of Family Growth. Vital Health Stat 23. December 2005;(25):1-160.
2. Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982-2010: data from the National Survey of Family Growth. Natl Health Stat Report. August 2013;(67):1-18.
3. Martinez G, Daniels K, Chandra A. Fertility of men and women aged 15-44 years in the United States: National Survey of Family Growth, 2006-2010. Natl Health Stat Report. 2012;(51):1-28.
4. Bushnik T, Cook J, Hughes E, Tough S. Seeking medical help to conceive. Health Rep. April 2012;23(4):7-13.
5. Rosenbaum TY, Owens A. The role of pelvic floor physical therapy in the treatment of pelvic and genital pain-related sexual dysfunction (CME). J Sex Med. 2008;5(3):513-523.
6. Sucher BM. Ultrasonography-guided osteopathic manipulative treatment for a patient with thoracic outlet syndrome. J Am Osteopat Assoc. 2011;111(9):543-547.
7. Boyles R, Toy P, Mellon J Jr, Hayes M, Hammer B. Effectiveness of manual physical therapy in the treatment of cervical radiculopathy: a systematic review. J Man Manip Ther. 2011;19(3):135-142.
8. Bayliss AJ, Klene FJ, Gundeck EL, Loghmani MT. Treatment of a patient with post-natal chronic calf pain utilizing instrument-assisted soft tissue mobilization: a case study. J Man Manip Ther. 2011;19(3):127-134.
9. Senbursa G, Baltaci G, Atay A. Comparison of conservative treatment with and without manual physical therapy for patients with shoulder impingement syndrome: a prospective, randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):915-921.
10. ?enbursa G, Baltaci G, Atay ÖA. The effectiveness of manual therapy in supraspinatus tendinopathy. Acta Orthop Traumatol Turc. 2011;45(3):162-167.
11. Gaspar PD, Willis FB. Adhesive capsulitis and dynamic splinting: a controlled, cohort study. BMC Musculoskelet Disord. September 2009;10:111.
12. Field DA, Miller S. Cosmetic breast surgery. Am Fam Physician. 1992;45(2):711-719.
13. Licciardone JC, Kearns CM, Hodge LM, Minotti DE. Osteopathic manual treatment in patients with diabetes mellitus and comorbid chronic low back pain: subgroup results from the OSTEOPATHIC Trial. J Am Osteopath Assoc. 2013;113(6):468-478.
14. Farrell JP, Jensen GM. Manual therapy: a critical assessment of role in the profession of physical therapy. Phys Ther. 1992;72(12):843-852.
15. Threlkeld AJ. The effects of manual therapy on connective tissue. Phys Ther. 1992;72(12):893-902.
16. Beastall GH, Ferguson KM, O’Reilly DS, Seth J, Sheridan B. Assays for follicle stimulating hormone and luteinising hormone: guidelines for the provision of a clinical biochemistry service. Ann Clin Biochem. 1987;24(pt 3):246-262.
17. Wurn BF, Wurn LJ, King CR, et al. Treating fallopian tube occlusion with a manual pelvic physical therapy. Altern Ther Health Med. 2008;14(1):18-23.
18. Wurn LJ, Wurn BF, King CR, Roscow AS, Scharf ES, Shuster JJ. Increasing orgasm and decreasing dyspareunia by a manual physical therapy technique. MedGenMed. 2004;6(4):47.
19. Wurn BF, Wurn LJ, Patterson K, King CR, Scharf ES. Decreasing dyspareunia and dysmenorrhea in women with endometriosis via a manual physical therapy: results from two independent studies. J Endometr. 2011;3(4):188-196.
20. Wurn BF, Wurn LJ, King CR. Treating female infertility and improving IVF pregnancy rates with a manual physical therapy technique. MedGenMed. 2004;6(2):51.
21. Wurn B, Wurn L, King R. Miracle Moms, Better Sex, Less Pain. Gainesville, FL: Med-Art Press; 2009.
22. Arnadottir TS, Sigurdardottir AK. Is craniosacral therapy effective for migraine? Tested with HIT-6 Questionnaire. Complement Ther Clin Pract. 2013;19(1):11-14.
23. Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Saavedra-Hernández M, Fernández-Sola C, Moreno-Lorenzo C. Effects of myofascial release techniques on pain, physical function, and postural stability in patients with fibromyalgia: a randomized controlled trial. Clin Rehabil. 2011;25(9):800-813.
24. Fryer G, Morse CM, Johnson JC. Spinal and sacroiliac assessment and treatment techniques used by osteopathic physicians in the United States. Osteopath Med Prim Care. April 2009;3:4.
25. Kramp ME. Combined manual therapy techniques for the treatment of women with infertility: a case series. J Am Osteopath Assoc. 2012;112(10):680-684.
26. Langenau EE, Dowling DJ, Dyer C, Roberts WL. Frequency of specific osteopathic manipulative treatment modalities used by candidates while taking COMLEX-USA Level 2-PE. J Am Osteopath Assoc. 2012;112(8):509-513.
27. Matarán-Peñarrocha GA, Castro-Sánchez AM, García GC, Moreno-Lorenzo C, Carreño TP, Zafra MD. Influence of craniosacral therapy on anxiety, depression and quality of life in patients with fibromyalgia. Evid Based Complement Alternat Med. 2011;2011:178769.
28. Moore SD, Laudner KG, McLoda TA, Shaffer MA. The immediate effects of muscle energy technique on posterior shoulder tightness: a randomized controlled trial. J Orthop Sports Phys Ther. 2011;41(6):400-407.
29. Rice AD, King R, Reed ED, Patterson K, Wurn BF, Wurn LJ. Manual physical therapy for non-surgical treatment of adhesion-related small bowel obstructions: two case reports. J Clin Med. 2013;2(1):1-12.
30. Wong CK. Strain counterstrain: current concepts and clinical evidence. Man Ther. 2012;17(1):2-8.
31. Bove GM, Chapelle SL. Visceral mobilization can lyse and prevent peritoneal adhesions in a rat model. J Bodyw Mov Ther. 2012;16(1):76-82.
32. Miles A. Science, humanism, judgement, ethics: person-centered medicine as an emergent model of modern clinical practice. Folia Med (Plovdiv). 2013;55(1):5-24.
33. Educational Council on Osteopathic Principles (ECOP) of the American Association of Colleges of Osteopathic Medicine (AACOM). Glossary of Osteopathic Terminology. Chevy Chase, MD: American Association of Colleges of Osteopathic Medicine; 2011. http://www.aacom.org/resources/bookstore/ Documents/GOT2011ed.pdf. Accessed June 6, 2014.
34. Altman DG. Practical Statistics for Medical Research. London, UK: Chapman and Hall; 1991.
35. Serafini P, Batzofin J. Diagnosis of female infertility: a comprehensive approach. J Reprod Med. 1989;34(1):29-40.
36. Sueoka K, Asada H, Tsuchiya S, Kobayashi N, Kuroshima M, Yoshimura Y. Falloposcopic tuboplasty for bilateral tubal occlusion: a novel infertility treatment as an alternative for in-vitro fertilization? Hum Reprod. 1998;13(1):71-74.
37. Rimbach S, Bastert G, Wallwiener D. Technical results of falloposcopy for infertility diagnosis in a large multicentre study. Hum Reprod. 2001;16(5):925-930.
38. Li SC, Liu MN, Hu XZ, Lu ZL. Hysteroscopic tubal catheterization and hydrotubation for treatment of infertile women with tubal obstruction. Chin Med J (Engl). 1994;107(10):790-793.
39. Thurmond AS, Machan LS, Maubon AJ, et al. A review of selective salpingography and fallopian tube catheterization. Radiographics. 2000;20(6):1759-1768.
40. Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
41. Bosteels J, Van Herendael B, Weyers S, D’Hooghe T. The position of diagnostic laparoscopy in current fertility practice. Hum Reprod Update. 2007;13(5):477-485.
42. Stepniewska A, Pomini P, Bruni F, et al. Laparoscopic treatment of bowel endometriosis in infertile women. Hum Reprod. 2009;24(7):1619-1625.
43. Zhu S, Liu D, Huang W, et al. Post-laparoscopic oral contraceptive combined with Chinese herbal mixture in treatment of infertility and pain associated with minimal or mild endometriosis: a randomized controlled trial. BMC Complement Altern Med. July 2014;14:222.
44. Lee HJ, Lee JE, Ku SY, et al. Natural conception rate following laparoscopic surgery in infertile women with endometriosis. Clin Exp Reprod Med. 2013;40(1):29-32.
45. Society for Assisted Reproductive Technology. Clinic summary report: all SART member clinics. 2010. https://www.sartcorsonline.com/rptCSR_PublicMultYear. aspx?ClinicPKID=0. Accessed January 10, 2013.
46. Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. April 2006;1:9.
47. Nelson LM. Clinical practice: primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
48. Bidet M, Bachelot A, Bissauge E, et al. Resumption of ovarian function and pregnancies in 358 patients with premature ovarian failure. J Clin Endocrinol Metab. 2011;96(12):3864-3872.
49. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544-551.
50. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745-2749.
51. Boyle J, Teede HJ. Polycystic ovary syndrome--an update. Aust Fam Physician. 2012;41(10):752-756.
52. Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. February 2011;3:25-35.
53. Nahuis MJ, Kose N, Bayram N, et al. Long-term outcomes in women with polycystic ovary syndrome initially randomized to receive laparoscopic electrocautery of the ovaries or ovulation induction with gonadotrophins. Hum Reprod. 2011;26(7):1899-1904.
54. Ayaz A, Alwan Y, Farooq MU. Efficacy of combined metformin-clomiphene citrate in comparison with clomiphene citrate alone in infertile women with polycystic ovarian syndrome (PCOS). J Med Life. 2013;6(2):199-201.
55. Lunde O, Djøseland O, Grøttum P. Polycystic ovarian syndrome: a follow-up study on fertility and menstrual pattern in 149 patients 15-25 years after ovarian wedge resection. Hum Reprod. 2001;16(7):1479-1785.
56. Amer SA, Li TC, Metwally M, Emarh M, Ledger WL. Randomized controlled trial comparing laparoscopic ovarian diathermy with clomiphene citrate as a first-line method of ovulation induction in women with polycystic ovary syndrome. Hum Reprod. 2009;24(1):219-225.
57. Ray A, Shah A, Gudi A, Homburg R. Unexplained infertility: an update and review of practice. Reprod Biomed Online. 2012;24(6):591-602.
58. Kamath MS, Bhattacharya S. Demographics of infertility and management of unexplained infertility. Best Pract Res Clin Obstet Gynaecol. 2012;26(6):729-738.
59. Brandes M, Hamilton CJ, van der Steen JO, et al. Unexplained infertility: overall ongoing pregnancy rate and mode of conception. Hum Reprod. 2011;26(2):360-368.
60. Jackson A. Reproductive system. In: Lin VW, Cardenas DD, Cutter NC, et al, eds. Spinal Cord Medicine: Principles and Practice. New York, NY: Demos Medical Publishing; 2003
61. Allahbadia GN, Merchant R. Fallopian tube recanalization: lessons learnt and future challenges. Womens Health (Lond Engl). 2010;6(4):531-548.
62. Gleicher N, Confino E, Corfman R, et al. The multicentre transcervical balloon tuboplasty study: conclusions and comparison to alternative technologies. Hum Reprod. 1993;8(8):1264-1271.
63. Rice AD, Patterson K, Wurn BF, King CR, Wurn LJ. Update on “Decreasing dyspareunia and dysmenorrhea in women with endometriosis via a manual physical therapy: results from 2 independent studies” [letter]. J Endometr. 2014;6(3):161-162.
64. Sharlip ID, Jarow JP, Belker AM, et al. Best practice policies for male infertility. Fertil Steril. 2002;77(5):873-882.
65. Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod. 1991;6(6):811-816.
All rights reserved. Terms and Conditions.

